October 2014 LIP of the Month
The CO2 sequestration potential of the ultramafic portions of Large Igneous Provinces
Siobhan A. Wilson and Simon M. Jowitt
School of Earth, Atmosphere and Environment, Monash University, Wellington Road, Clayton, Melbourne, VIC 3800, Australia
Email: sasha.wilson@monash.edu; simon.jowitt@monash.edu
Carbon mineralization in mafic and ultramafic rocks
Carbon mineralization, the storage of CO2 within the crystal structures of carbonate minerals, is recognised as one of the safest and most effective methods for controlling the rising concentrations of atmospheric greenhouse gases that are associated with anthropogenic climate change (Seifritz, 1990; Lackner et al., 1995; Lackner, 2003). Unlike traditional Carbon Capture and Storage (CCS), which relies primarily upon stratigraphic trapping of injected supercritical CO2 in sedimentary rock formations, carbon mineralization technologies employ direct production of carbonate minerals to trap and store CO2. Sequestration of CO2 within carbonate minerals is typically achieved by reaction of this greenhouse gas with Mg- and Ca-silicate and hydroxide minerals such as forsterite, serpentine minerals and brucite. The lower reactivity of pyroxenes and other highly polymerized silicate minerals limits the utility of intermediate to felsic rocks as feedstocks for carbon mineralization. However, abundant natural deposits of mafic and ultramafic rocks represent an important resource for managing anthropogenic greenhouse gas emissions.
Carbon mineralization technologies (recently reviewed by Power et al., 2013) are focused on (1) in situ strategies that rely upon injection of CO2 into mafic and ultramafic geological formations and (2) ex situ technologies that require extraction of ultramafic minerals via mining (or generation of alkaline waste materials by industry). Ex situ carbonation includes carbonation in high-temperature and high-pressure reactors to capture industrial CO2 emissions as well as enhanced weathering of mineral wastes generated by mining and other industries for direct capture of CO2 from the atmosphere or industrial point sources.
Here, we discuss the potential for natural and enhanced CO2 sequestration in Large Igneous Provinces (LIPs), with a focus on the ultramafic portions of LIPs. Mafic rocks are an order of magnitude more common at Earth’s surface than ultramafic rocks and, although they carbonate more slowly, they represent a substantial resource for CO2 sequestration within LIPs. Trapping and mineralizing CO2 either within basalts or in basalt-capped reservoirs, including continental flood basalts (CFBs) formed during mafic-ultramafic LIP events (e.g. McGrail et al., 2006), has the potential to offset anthropogenic CO2 emissions. For example, Matter and Kelemen (2009) and Gislason et al. (2010) report that basalts of the Columbia River LIP have the potential to host large quantities of CO2 (between 36 and 148 Gt), primarily within low permeability basalts of the LIP that are vesicular and brecciated. The technology required to carbonate basalts in situ (i.e., without extracting basalt for use in reactors) is already being developed and tested. The CarbFix pilot project is now injecting CO2 charged waters directly into basaltic rocks in southwest Iceland (e.g., Gislason et al., 2010; www.or.is/en/projects/carbfix) and the Big Sky Carbon Sequestration Partnership has been injecting CO2 into the Columbia River Basalt near Wallula, WA, USA (http://www.bigskyco2.org/research/geologic/basaltproject).
Ultramafic rocks are ideal feedstocks for carbon mineralization because they (1) contain a high proportion of reactive forsterite and serpentine (>80 wt.% in many cases) and (2) produce durable Mg-carbonate minerals as reaction products. Recent research has demonstrated significant sequestration of atmospheric CO2 by ultramafic rocks and particularly by mineral wastes associated with ore deposits within ophiolite sequences (e.g. Wilson et al., 2009; Power et al., 2009, 2014; Pronost et al., 2012; Beinlich and Austrheim, 2012; Oskierski et al., 2013) and komatiitic lavas within Archean LIPs (Wilson et al., 2014). Carbonation of Mg-silicate minerals in ophiolitic outcrop (and basalt as well) is thermodynamically favoured and proceeds via reaction with atmospheric CO2 dissolved in rainwater and alkaline spring waters. The rate of CO2 sequestration is readily enhanced, by over two orders of magnitude, in the finely pulverised mineral wastes generated by ultramafic-hosted ore deposits (Wilson et al., 2009; Power et al., 2013).
The capacity of ultramafic rocks to fix CO2 is significant. Kelemen and Matter (2008) propose a strategy for in situ carbonation of ophiolites whereby buried ultramafic rocks are accessed by drilling, hydraulic fracture and injection of supercritical CO2. They suggest that total carbonation of peridotite within the Semail ophiolite of Oman could sequester 30 trillion tons of CO2, indicating that although total carbonation is highly unlikely, ophiolitic rocks could sequester significant volumes of CO2. For perspective, current global CO2 emissions are estimated at 38 Gt per annum, with 76% of emissions resulting from combustion of fossil fuels (IPCC, 2007). Therefore, complete carbonation of the ultramafic rocks within the Semail ophiolite alone would effectively offset global CO2 emissions for nearly 800 years. However, despite the extensive research that has been undertaken on the CO2 sequestration potential of ophiolites and CFBs, comparatively little has been done on the CO2 sequestration potential of the ultramafic portions of LIPs.
LIPs as a resource for reactive ultramafic rocks
The magmatism that generates LIPs can produce large volumes of ultramafic rocks, although the volumes of ultramafic rock within individual LIPs vary significantly (e.g., Ernst, 2014). For instance, the Siberian Trap, Deccan Trap and Columbia River LIPs are dominated by CFBs, whereas the Bushveld LIP, which includes the world’s largest mafic to ultramafic intrusive complex, contains significant amounts of ultramafic rock. Importantly, even CFB-dominated LIPs can contain both ultramafic intrusions and ultramafic (i.e. picritic) basalts. This is exemplified by the Muskox intrusion associated with the 1.27 Ga Mackenzie LIP of northern Canada (Fig. 1), which is dominated by a ~2,400-km long dyke swarm. The Muskox intrusion itself, although comprising a relatively small volume of the LIP, is ~125-km long, may extend another 250 km under cover (e.g. Barnes and Francis, 1995), thins from a width of 11 km to 100 m and is divided into feeder, marginal zones, a central layered series and an upper border group, with the first three of these subdivisions being dominated by or containing ultramafic rocks (e.g. Smith and Kapp, 1963).
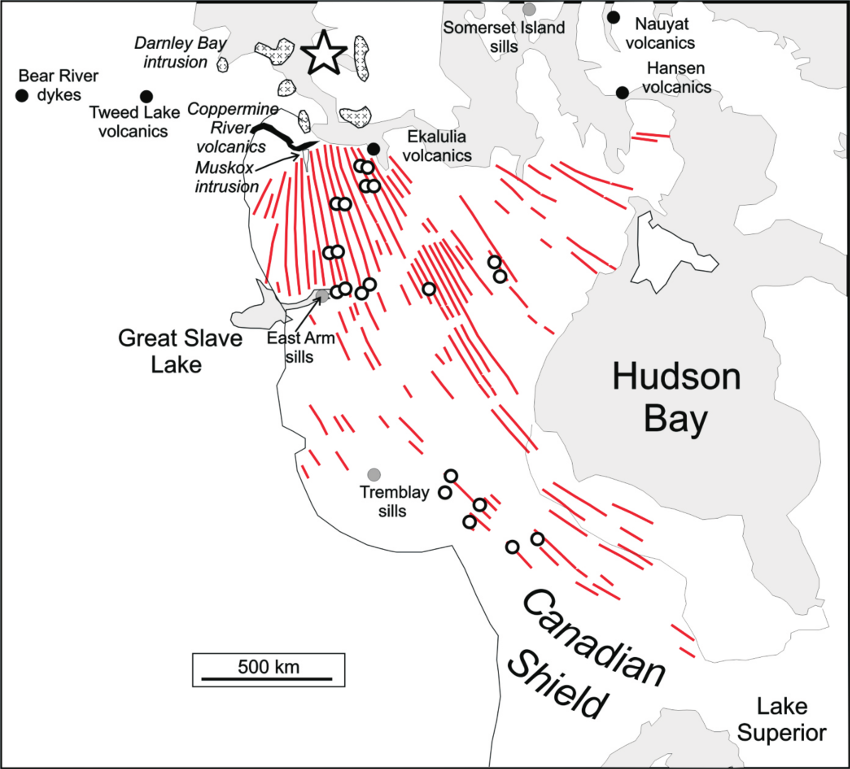
Fig. 1. Location of the Muskox intrusion within the Mackenzie LIP, showing that even basalt-dominated LIP events can contain significant amounts of ultramafic rocks. Modified from Jowitt and Ernst (2013) and showing samples sites (open circles) in that study.
Other examples of LIPs that contain significant volumes of ultramafic rocks include the Thompson area of the circum-Superior LIP and various Archean LIPs or LIP analogues (e,g., Ernst, 2014). Importantly, Archean LIPs, such as the magmatism within the Eastern Goldfields Superterrane of the Yilgarn Craton of Western Australia (i.e. the Eastern Yilgarn LIP), are typically associated with voluminous ultramafic komatiites, as well as often hosting significant mafic–ultramafic layered intrusions, such as the ~30,000 km3 Windimurra Complex of Western Australia (Ivanic et al., 2010, 2012). Many komatiite-hosted ore deposits are currently being mined in the Yilgarn Craton, including the Mount Keith Nickel Mine in the Agnew–Wiluna Greenstone Belt (Fig. 2). A recent study demonstrates that, unbeknownst to the mine operator, Mount Keith has been offsetting 11% of its annual greenhouse gas emissions via weathering and carbonation of its mine tailings (Wilson et al., 2014). Changes to tailings management and ore processing practises could be made to further reduce – or completely offset – the emissions produced by Mount Keith and many other mines (Harrison et al., 2013; Wilson et al., 2014). The CO2 sequestration potential of LIP intrusions is also being actively investigated, with research currently underway to make use of pyroxene-rich mafic tailings from PGM mines in the Bushveld LIP as a feedstock for industrial carbonation reactors (e.g., Meyer et al., 2014).
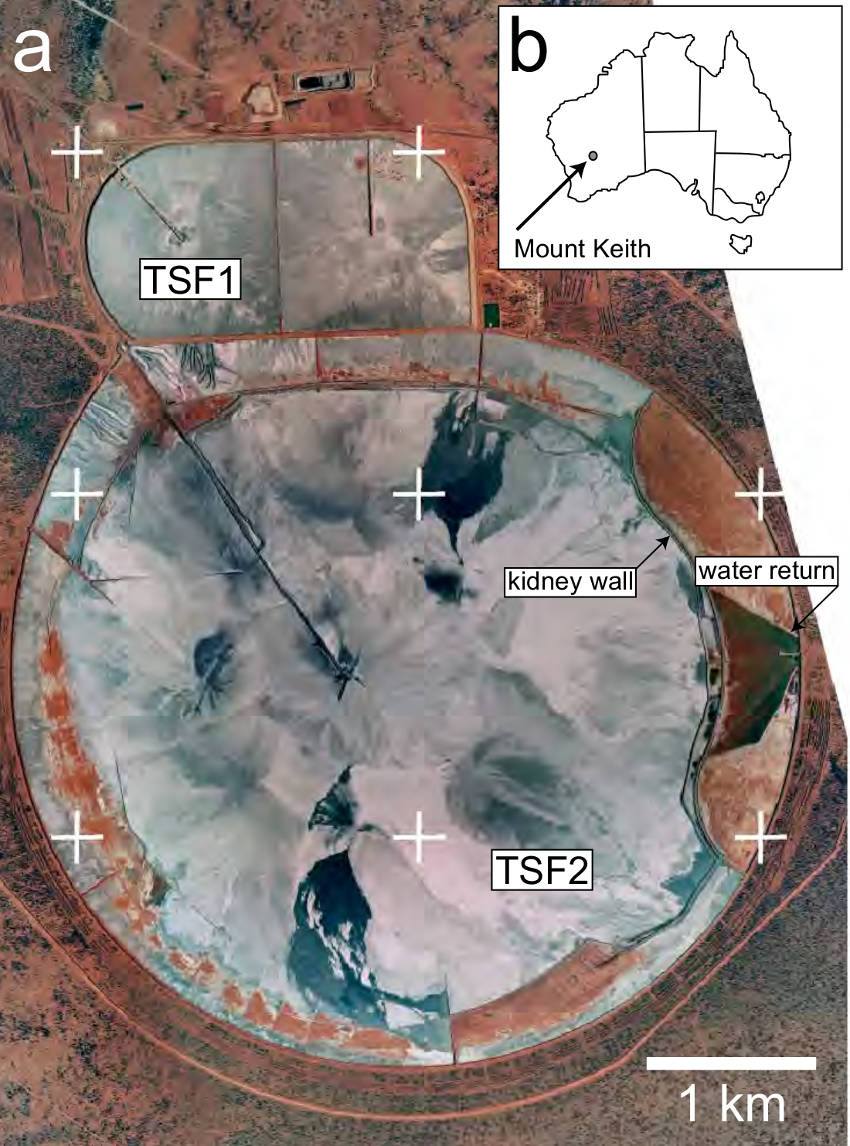
Fig. 2. (a) Location of the Mount Keith Nickel Mine in the Yilgarn Craton of Western Australia. (b) The tailings storage facilities (TSF1 and TSF2) at Mount Keith. TSF2 alone captures approximately 39,900 tonnes of CO2 from the atmosphere each year. Modified from Wilson et al. (2014).
If and when carbon mineralization technologies become widely implemented, it will become important to accurately determine the amount ultramafic rocks suitable for CO2 sequestration that are present in LIPs as well as ophiolites. The quantification of the abundance of ultramafic rocks within a given LIP ranges from good (e.g. in the case of the Bushveld LIP, which is dominated by a single, gigantic, well-studied and relatively uniform layered intrusion) to poor. Nevertheless, the level of quantification is improving; this is demonstrated by recent research within the Wrangellia LIP, where geophysical 3D modelling of a ~7 x ~5 km area of the Amphitheater Mountains in Alaska identified 2,000 km3 of ultramafic rocks in the subsurface (to a depth of 4 km; Glen et al., 2011). The results of the latter study indicate that research primarily focused on Ni-Cu-PGE exploration can also feed into determining the CO2 sequestration potential of the ultramafic portions of LIP events. Furthermore, even in CFB-dominated LIPs, the ultramafic rocks that are most valuable for CO2 sequestration represent volumetrically significant portions of the rocks formed during these events. For example, the Wrangellia LIP hosts an estimated ~3,500 km2 of ultramafic rock within 4 km of the Earth’s surface versus a currently exposed area of ~27,000 km2 (Greene et al., 2010) and a total estimated volume of ~1 million km3 (Ernst and Buchan, 2001). All of this demonstrates that even CFB-dominated LIP events can produce voluminous ultramafic rocks of significant value for sequestration of CO2.
Conclusions
Although the basaltic portions of LIPs have long been recognised as a resource for CO2 sequestration via carbon mineralization, the CO2 sequestration potential of the ultramafic components of these LIP events remains understudied. Archean LIPs, komatiite-dominated sections of LIPs (e.g. the Thompson Belt), and layered intrusion-dominated LIPs (e.g., the Bushveld LIP) in particular represent a relatively unexplored and untapped potential resource for CO2 sequestration. Thus, increased efforts should be made to estimate the size of this resource and to take better advantage of the CO2 sequestration potential of these and many other LIPs.
References
Barnes, S. J. and Francis, D. (1995) The distribution of platinum-group elements, nickel, copper, and gold in the Muskox layered intrusion, Northwest Territories, Canada. Economic Geology 90, 135–154.
Beinlich, A. and Austrheim, H. (2012) In situ sequestration of atmospheric CO2 at low temperature and surface cracking of serpentinized peridotite in mine shafts. Chemical Geology 323–333, 32–44.
Ernst, R.E. (2014). Large Igneous Provinces. Cambridge University Press, 653 p,
Ernst, R.E., and Buchan, K.L. (2001) Large mafic magmatic events through time and links to mantle-plume heads, in: Ernst, R.E., and Buchan, K.L. (Eds.), Mantle Plumes: Their Identification Through Time, Geological Society of America Special Paper 352, 483–57
Glen, J. M., Schmidt, J. M., and Connard, G. G. (2011) Three–dimensional model of an ultramafic feeder system to the Nikolai Greenstone mafic large igneous province, central Alaska Range. Geochemistry, Geophysics, Geosystems 12, DOI: 10.1029/2011GC003508.
Gislason, S.R., Wolff-Boenisch, D., Stefansson, A., Oelkers, E.H., Gunnlaugsson, E., Sigurdardottir, H., Sigfusson, B., Broecker, W.S., Matter, J.M., and Stute, M. (2010) Mineral sequestration of carbon dioxide in basalt: A pre-injection overview of the CarbFix project. International Journal of Greenhouse Gas Control 4, 537–545.
Greene, A. R., Scoates, J. S., Weis, D., Katvala, E. C., Israel, S., and Nixon, G. T. (2010). The architecture of oceanic plateaus revealed by the volcanic stratigraphy of the accreted Wrangellia oceanic plateau. Geosphere 6, 47–73.
Harrison, A.L., Power, I.M. and Dipple, G.M., 2013. Accelerated carbonation of brucite in mine tailings for carbon sequestration. Environmental Science & Technology 47, 126–134.
IPCC (2007) IPCC Climate Change 2007: Synthesis Report, An Assessment of the Intergovernmental Panel on Climate Change, http://www.ipcc.ch/pdf/assessment-report/ar4/syr/ar4_syr.pdf.
Ivanic, T.J., Wingate, M.T.D., Kirkland, C.L., Van Kranendonk, M.J., and Wyche, S. (2010). Age and significance of voluminous mafic–ultramafic magmatic events in the Murchison Domain, Yilgarn Craton. Australian Journal of Earth Sciences 57, 597–614.
Ivanic, T.J., Van Kranendonk, M.J., Wingate, M.T.D., Kirkland, C.L., and Wyche, S. (2012). October 2012 LIP of the Month. http://www.largeigneousprovinces.org/12oct, accessed 28/10/14
Jowitt, S. M., and Ernst, R. E. (2013). Geochemical assessment of the metallogenic potential of Proterozoic LIPs of Canada. Lithos 174, 291–307.
Kelemen, P.B. and Matter, J. (2008) In situ carbonation of peridotite for CO2 storage. Proceedings of the National Academy of Sciences of the USA 105, 17295–17300.
Lackner, K.S. (2003) Climate change - A guide to CO2 sequestration. Science 300, 1677–1678.
Lackner, K.S., Wendt, C.H., Butt, D.P., Joyce, E.L. and Sharp, D.H. (1995) Carbon dioxide disposal in carbonate minerals. Energy 20, 1153–1170
Matter, J.M. and Kelemen, P.B. (2009) Permanent storage of carbon dioxide in geological reservoirs by mineral carbonation. Nature Geoscience 12, 837–841.
McGrail, B.P., Schaef, H.T., Ho, A.M., Chien, Y.-J., Dooley, J.J., and Davidson, C.L. (2006) Potential for carbon dioxide sequestration in flood basalts. Journal of Geophysical Research 111, 1–13.
Meyer, N.A., Vögeli, J.U., Becker, M., Broadhurst, J.L., Reid, D.L., and Franzidis, J.-P. (2014) Mineral carbonation of PGM mine tailings for CO2 storage in South Africa: A case study. Minerals Engineering 59 45–51.
Oskierski, H.C., Dlugogorski, B.Z., and Jacobsen, G., 2013. Sequestration of atmospheric CO2 in chrysotile mine tailings of the Woodsreef Asbestos Mine, Australia: Quantitative mineralogy, isotopic fingerprinting and carbonation rates. Chemical Geology 358, 156–169.
Power, I.M., Wilson, S.A., Harrison, A.L., Dipple, G.M., McCutcheon, J., Southam, G., and Kenward, P.A. (2014) A depositional model for hydromagnesite–magnesite playas. Sedimentology 61, 1701–1733.
Power, I.M., Harrison, A.L., Dipple, G.M., Wilson, S.A., Kelemen, P.B., Hitch, M., and Southam, G. (2013) Carbon mineralization: From natural analogues to engineered systems. Geochemistry of Geologic CO2 Sequestration. DePaolo, D., Cole, D., Bourg, I., and Navrotsky, A. (Eds.). Reviews in Mineralogy and Geochemistry 77, 305–360.
Power, I.M., Wilson, S.A., Thom, J.M., Dipple, G.M., Gabites, J.E., and Southam, G. (2009) The hydromagnesite playas of Atlin, British Columbia, Canada: A biogeochemical model for CO2 sequestration. Chemical Geology 260, 286–300.
Pronost, J., Beaudoin, G., Lemieux, J.-M., Hebert, R., Constantin, M., Marcouiller, S., Klein, M., Duchesne, J., Molson, J.W., Larachi, F., and Maldague, X. (2012) CO2-depleted warm air venting from chrysotile milling waste (Thetford Mines, Canada): Evidence for in-situ carbon capture from the atmosphere. Geology 40, 275–278.
Seifritz, W., 1990. CO2 disposal by means of silicates. Nature 345, 486.
Smith, C. H., and Kapp, H. E. (1963). The Muskox intrusion, a recently discovered layered intrusion in the Coppermine River area, Northwest Territories, Canada. Special Paper of the Mineralogical Society of American 1, 30–35.
Wilson, S.A., Harrison, A.L., Dipple, G.M., Power, I.M., Barker, S.L.L., Mayer, K.U., Fallon, S.J., Raudsepp, M., and Southam, G. (2014) Offsetting of CO2 emissions by air capture into mine tailings at the Mount Keith Nickel Mine, Western Australia: Rates, controls and prospects for carbon neutral mining. International Journal of Greenhouse Gas Control 25, 121–140.
Wilson, S.A., Dipple, G.M., Power, I.M., Thom, J.M., Anderson, R.G., Raudsepp, M., Gabites, J.E., and Southam, G. (2009) Carbon dioxide fixation within mine wastes of ultramafic-hosted ore deposits: Examples from the Clinton Creek and Cassiar chrysotile deposits, Canada. Economic Geology 104, 95–112.
